The earth has been bathing in sunlight for more than 3 billion years. The sun produces an enormous amount of energy including cosmic rays, gamma rays, x-rays, ultraviolet radiation, visible radiation and infrared radiation. Ordinarily, sunlight is broken down into three major components:
(1) visible light, with wavelengths between 0.4 and 0.8 micrometre,
(2) ultraviolet light, with wavelengths shorter than 0.4 micrometre, and
(3) infrared radiation, with wavelengths longer than 0.8 micrometre.

Most of the ultraviolet radiation is efficiently absorbed by the stratospheric ozone layer; however, some reaches the earth, and with other penetrating solar rays, is essential to life and optimal health. Although ultraviolet light constitutes only a very small proportion of the total radiation that reaches the earth’s surface, this component is extremely important for our health. It produces vitamin D through the activation of ergosterol and plays a role in several other important bioregulatory processes in the body.
The healing power of the sun and its use in medical treatment (heliotherapy) have roots extending back into antiquity. Many traditional medical systems (Egyptian, Greek, Romans, Chinese, etc.) have recognized sunlight as a therapeutic force for millennia. In the modern era, particularly the first half of the 20th century, heliotherapy was widely used in both Europe and North America.
Cultural Changes Affecting Sunlight Exposure
Over time, clothing became the norm in higher latitudes and then eventually a social attribute in many societies. By the 1600s, peoples in these regions covered their whole body, even in summertime. As the industrial revolution swept across Northern Europe in the early 19th century, physicians began reporting that children living in the inner cities of Glasgow and London were developing skeletal deformities especially prominent in the legs as well as growth retardation. By the turn of the 19th century, it was estimated that more than 90% of children living in the industrial cities throughout Europe had this bone deformity disease known as rickets.1
In 1822, Sniadecki reported that children living in Warsaw were afflicted with rickets whereas children living in the rural areas outside of Warsaw did not develop this bone deformity disease. He concluded, “strong and obvious is the influence of sun on the cure of rickets and the frequent occurrence of the disease in densely populated towns where the streets are narrow and poorly lit”.2
It was inconceivable to the medical community of that time how exposure of the skin to sunlight could have any health consequences on the skeleton or disease. Consequently, this observation was ignored for almost 100 years.

Typical living conditions in the Gorbals in 1912. This region of Glasgow was the most notorious slum in the United Kingdom. (Mitchell Library, Glasgow Life)
In 1890, Theobald Palm, a medical missionary, wrote to his colleagues living in India and China where nutrition was extremely poor, asking whether they were seeing children with rickets. They reported that it was a rare condition in these countries. He reasoned that children living in London had better nutrition and better housing conditions, and therefore the only common denominator was that children living in the polluted cities of London and Glasgow were not exposed to adequate sunlight. He encouraged sunbathing as a method to treat and prevent rickets. While Palm’s observations were in some ways anecdotal, they had a potent effect on the development of photobiology.3
About that same time in 1893, Niels Ryberg Finsen published a paper about the effects of light on skin. Finsen suffered from Niemann-Pick disease, and noted that his own sluggishness seemed cured with a regular daily dose of sunlight. This inspired him to investigate the effects of light on living things. Finsen discovered that certain wavelengths of light can generate healing properties and was eventually able to demonstrate its effects on a skin condition called lupus vulgaris. He also showed that solar radiation could help treat smallpox and tuberculosis. Finsen won the Nobel Prize in Physiology in 1903 for his work on phototherapy.4, 5, 6

In 1895, Finsen founded the Finsen Institute for Phototherapy in Copenhagen, which was dedicated to studying the effects of light or phototherapy on health conditions. There were at least 800 lupus vulgaris patients treated at the Institute. Of those patients, at least half were cured.
Doctors throughout Europe and North America began promoting whole-body sunbathing to help prevent rickets. It was also recognized that wintertime sunlight in the temperate zone was too feeble to prevent rickets. For this reason, many children were exposed to UVR from a mercury or carbon arc lamp for one hour three times a week, which proved to be an effective preventive measure and treatment.
Around the time the solar solution to rickets gained widespread recognition in medical circles, another historic scourge, tuberculosis (TB), was also found to respond to solar intervention. TB patients of all ages were sent to rest in sunny locales and generally returned in good health.

Sunlight therapy, or heliotherapy, became even more popular after a Swiss doctor, Auguste Rollier, began using it in the early 1900s. Inspired by Finsen, Rollier enthusiastically opened “solaria” throughout Switzerland.7 These were buildings designed to optimize exposure to the sun’s rays. Soon, buildings, all with south-facing balconies and some with sliding walls of windows and retractable roofs, were built across Europe. Rollier devised a detailed protocol for how, exactly, to sunbathe for health. He was convinced that early-morning sun was best, and that sun exposure was most beneficial when the air was cool. When patients, most of whom had tuberculosis, arrived at his solaria, they first had to adjust to the altitude (his clinics were in the mountains), and then to the cool air. Once acclimated, Rollier slowly exposed them to the sun. 8, 9
Soon doctors across Europe were advocating heliotherapy as a cure for all sorts of afflictions, such as infectious diseases, wounds, burns, arthritis, rheumatism and nerve damage. Suntans became popular, and many proclaimed the sun to be the long-sought fountain of youth.

By this time, scientists had accumulated evidence for the practice as well. Researchers showed that sunlight could kill the bacteria that caused tuberculosis and other infectious diseases.10 Others later proved that ultraviolet light could cure rickets, by creating vitamin D on the skin.11

Vitamin D and Sunlight
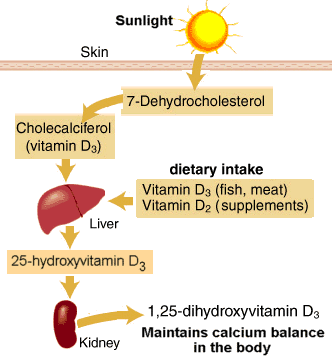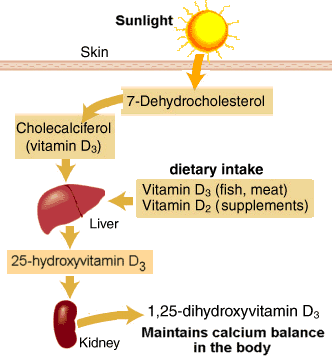
Human skin contains a compound that functions as a precursor to vitamin D, called 7-dehydrocholesterol. When ultraviolet light from the sun penetrates the skin, a specific portion of the light can transform 7-dehydrocholesterol into vitamin D3 by breaking a bond in the precursor molecule. Specifically, it’s the ultraviolet-B portion of the spectrum, which moves at a faster wavelength than its slower cousin, ultraviolet-A rays. Simply put, sunlight breaks a bond in a molecule on the skin and then the body uses the new, sun-altered compound for biochemical purposes. The best-known benefit of sunlight is its ability to boost the body’s vitamin D supply. Most cases of vitamin D deficiency are due to lack of outdoor sun exposure.
At least 1,000 different genes governing virtually every tissue in the body are now thought to be regulated by 1,25-dihydroxyvitamin D3(1,25[OH]D), the active form of the vitamin, including several involved in calcium metabolism and neuromuscular and immune system functioning. The efficiency of production depends on the number of ultraviolet-B photons that penetrate the skin, a process that can be curtailed by clothing, excess body fat, sunscreen, and the skin pigment melanin.
Over the past three decades, several thousand articles have been published about the health benefits of sunlight exposure and optimal vitamin D status via ultraviolet exposure, diet and supplements. Observations have been supported by retrospective studies that link low circulating levels of 25-hydroxyvitamin D, a measure of vitamin D status, with an increased risk of a vast array of detrimental conditions, including type 2 diabetes mellitus, infectious diseases, cancer, multiple sclerosis and neurocognitive dysfunction.
By World War II, the popularity of sunbathing and heliotherapy gradually declined. Newly discovered antibiotics were thought more powerful against germs than sunlight. The era of pharmaceuticals was in full bloom by the 1950s, and heliotherapy was virtually forgotten.
Phototherapy
Over the last century, as sunbathing became less popular, phototherapy continued to play a pivotal role in the treatment of dermatologic diseases. In the middle of the 20th century, advancements in ultraviolet-B light therapy expanded treatment options for patients with psoriasis. Now, phototherapy, either as monotherapy or combined with other modalities, is used increasingly in the outpatient management of inflammatory dermatoses such as psoriasis, keratinizing disorders such as pityriasis lichenoides chronica and keratosis pilaris, and the eczemas, particularly atopic dermatitis.
In the 1970s, photochemotherapy (i.e. using psoralen as a photosensitizer in combination with ultraviolet-A radiation [PUVA]) made its debut. PUVA became established in the treatment of skin diseases in the last quarter of the 20th century. More recent advances in the past few decades that have revolutionized phototherapy include narrowband ultraviolet-B, laser and other targeted phototherapy, and photodynamic therapy. Another new light source, UVA1, has been developed in the last decade. In the absence of adequate therapeutic action spectra research data, the empirical use of UVA1 (340-400 nm) has shown these wavelengths to be beneficial in the treatment of atopic dermatitis. Two types of UVA1 emission sources currently are in use: a lower dose output fluorescent tube, and a high output filtered metal halide source, particularly popular in Germany, with treatments of up to 130 joules UVA/cm2 possible.
The Advent of Sun Avoidance
Nowadays, the sundial of heliotherapy has moved in the opposite direction. Most public health messages of the last several decades have focused on the hazards of too much sun exposure. Exposure to sunlight is actively discouraged for fear of skin cancer, and contemporary lifestyles are associated with long hours spent under artificial light indoors.
Ultraviolet radiation penetrates deeply into the skin, where it can contribute to skin cancer indirectly via generation of DNA-damaging molecules such as hydroxyl and oxygen radicals. Both forms of ultraviolet light (A and B) can damage collagen fibers, accelerate aging of the skin, and increase the risk of skin cancers. Excessive sun exposure can also cause cataracts and diseases aggravated by UV radiation-induced immunosuppression such as reactivation of some latent viruses.

The World Health Organization's International Agency for Research on Cancer recommends avoiding outdoor activities at midday, wearing clothing to cover the whole body, and daily use of sunscreen on usually exposed skin.12 Several organizations began advocating "Slip, Slop, Slap, Seek, Slide." “Slip on a shirt, Slop on the 50+ sunscreen, Slap on a hat, Seek shade or shelter, Slide on some glasses used to block out sun.”13 The U.S. Surgeon General issued a Call to Action focused on reducing ultraviolet exposure, whether from indoor ultraviolet or from the sun.14 These recommendations are understandable from the viewpoint of preventing new cases of skin cancer each year, but they neglect the fact that we have a long cross-cultural history of appreciation of the sun and use of the sun’s radiation for healing purposes.
Many dermatologists now recommend spending small amounts of time in the sun without sun protection to ensure adequate production of vitamin D. Adequate amounts can be produced with moderate sun exposure to the face, arms and legs, averaging 10 to 30 minutes several times per week without sunscreen. People with darker skin may need a little more than this. Exposure time should depend on how sensitive your skin is to sunlight. Vitamin D levels should be periodically checked, and D-3 supplementation may be necessary, even with moderate sun exposure.
While there is incontrovertible evidence that ultraviolet radiation is a significant predisposing factor for skin cancers15, a growing body of data suggest numerous general health benefits are brought about by sunlight. Particularly, the fact that increased sun exposure has been associated with protection from several different types of cancer16, 17, 18, 19, 20, 21, 22, 23, 24, 25, diabetes26, multiple sclerosis27, and numerous other dseases.28, 29, 30, 31 In fact, the current policy of sun avoidance is creating probable harm for the general population.
Sun exposure opinions are slowly changing. The National Academy of Sciences recently assembled an international group of medical experts from different fields to discuss sun safety. The 2018 report from that meeting, published in JAMA Dermatology, stated that “although the harms associated with overexposure outweigh the benefits, the beneficial effects of ultraviolet radiation exposure should not be ignored in developing new sun safety guidelines.”32
Sunlight and Nitric Oxide
Research now shows that sunlight produces physiological responses well beyond the production of vitamin D. Specifically, it is observed that when the skin is exposed to the ultraviolet-A portion of the solar spectrum, which ranges from 315 nm to 400 nm, nitric oxide (NO) is released in the body. Nitric oxide is a potent vasodilator and is a widespread signaling molecule that participates in virtually every cellular and organ function in the body. It is involved in the maintenance of vascular tone, neurotransmitter function in both the central and peripheral nervous systems, and mediation of cellular defense. In addition, NO interacts with mitochondrial systems to regulate cell respiration and to augment the generation of reactive oxygen species, thus triggering mechanisms of cell survival or death. Thus, when the skin is stimulated with ultraviolet-A radiation, nitric oxide is released, stimulating vasodilation and lowering blood pressure.33
A 2014 study showed that during active exposure to ultraviolet-A, diastolic blood pressure fell by roughly 5 mm Hg and remained lower for 30 minutes after exposure.33 Several other studies have also demonstrated that exposure with ultraviolet-A leads to a sustained reduction in blood pressure.34, 35, 36, 37
This is an important finding as small changes in population blood pressure can produce significant reductions in deaths from cerebral and coronary vascular disease. The fall in mortality due to stroke, ischemic heart disease, and other vascular diseases is directly and linearly proportional to the degree of reduction in blood pressure. Even a 20 mmHg lower systolic blood pressure leads to a two-fold reduction in overall mortality in both men and women aged 40-69 years.38 Additionally, a reduction of diastolic blood pressure by 5 mm Hg decreases risk for stroke by 34% and coronary heart disease by 21%.39
A 2014 study that looked at the sun habits of nearly 30,000 women in Sweden over the course of 20 years found that women who avoided the sun had a mortality rate twice as high as those who got a lot of sun exposure. This study found that all‐cause mortality was inversely related to sun exposure habits in a “dose-dependent” manner. The mortality rate was increased twofold amongst avoiders of sun exposure as compared to those with the highest sun exposure habits.40
Other Beneficial Physiological Effects of Sunlight
Aside from the production of vitamin D and nitric oxide, there are many other sunlight-mediated effects on physiology. Numerous cells are closely associated with cutaneous (skin) nerve fibers, or the “neuro‐immuno‐cutaneous system”, which through the action of neuropeptides can modulate cellular function. Under the impact of ultraviolet radiation virtually all properties of the neuro‐immuno‐cutaneous system are modified. In the human skin, melanogenesis (the development of melanin) is initiated by exposure to ultraviolet radiation, causing the skin to darken. Melanin is an effective absorbent of light as this pigment can dissipate over 99.9%of absorbed ultraviolet radiation. Because of this property, melanin is thought to protect skin cells from ultraviolet-B radiation damage, reducing the risk of skin damage and skin cancer.
Ultraviolet radiation not only induces melanin synthesis, it can also stimulate the production of certain neuropeptides, especially alpha-melanocyte-stimulating hormone (α-MSH) and calcitonin gene-related peptide (CGRP). Thus, upon exposure to sunshine, melanocytes and keratinocytes in the skin release α-MSH and CGRP, which have been shown to suppress autoimmune diseases and reestablish tolerance to autoantigens.41 α-MSH also helps limit oxidative DNA damage resulting from ultraviolet radiation and increases gene repair, thus reducing melanoma cancer risk.42
Calcitonin gene-related peptide (CGRP) is a protein in the brain and nervous system involved in the transmission of pain and the resultant reaction of tissues and blood vessels. This peptide is considered to play a positive role in wound healing and protects against vascular ischemia (restriction in blood supply to tissues) and tissue traumas. Interestingly, it is released in response to both ultraviolet-A and ultraviolet-B exposure. This neuropeptide also can modulate several overexpressed cytokines and thus can help control certain autoimmune diseases. The release of this peptide can help explain in part sunlight’s efficacy in treating skin disorders such as psoriasis.43
Circadian Rhythms (Chronobiology) and Melatonin
From sunflowers to sparrows, zebras to humans, everything under the sun follows the pattern of the sun. In 1729, French scientist Jean-Jacques d’Ortous de Mairan recorded the first observation of an endogenous, or built-in, circadian oscillation in the leaves of the “shame plant” Mimosa pudica.44 Even in total darkness, the plant continued its daily rhythms. This led to the conclusion that the plant was not simply relying on external cues, or “zeitgebers”, but also its own internal biological clock. As daylight or diurnal creatures, we humans are programmed to be outdoors while the sun is shining and home in bed at night. This light-dark cycle creates a biological regulatory circadian oscillation. Decades of research in chronobiology show that the timing, intensity and duration of exposure to daylight and darkness directly affect how well people sleep, how well they function while awake, and how well they feel.
The hormone melatonin (N-acetyl-5-methoxytryptamine) is manufactured in the pineal gland and is associated with numerous physiological functions. Typically, melatonin levels start to rise in the mid-to-late evening (after the sun has set), and stay elevated for most of the night. Then melatonin drops in the early morning as the sun rises, and cortisol increases. Thus, melatonin is secreted at night in response to darkness and is thereby associated with sleep, lowered core body temperature, and other nighttime physiological events. The period of melatonin secretion has been described as “biological night”.

Light exposure to the retina is relayed via the suprachiasmatic nucleus (in the hypothalamus) and inhibits melatonin secretion. Melatonin is secreted in response to periods of darkness, resulting in higher concentrations at night.
When people are exposed to sunlight or very bright artificial light in the morning, their nocturnal melatonin production occurs sooner, and they enter sleep more easily at night. Melatonin production also shows a seasonal variation relative to the availability of light, with the hormone produced for a longer period in the winter than in the summer.


This pineal hormone is a key pacesetter for many of the body’s circadian rhythms. It also plays an important role in countering infection, inflammation, cancer, and autoimmunity. The melatonin rhythm phase caused by exposure to bright morning light has been effective against insomnia, premenstrual syndrome, and seasonal affective disorder (SAD).

The melatonin precursor, serotonin, is also affected by exposure to daylight. Normally produced during the day, serotonin is only converted to melatonin in darkness. Whereas high melatonin levels correspond to long nights and short days, high serotonin levels in the presence of melatonin reflect short nights and long days. Moderately high serotonin levels result in more positive moods and a calm yet focused mental outlook. Indeed, SAD has been linked with low serotonin levels during the day as well as with a phase delay in nighttime melatonin production. It was recently found that mammalian skin can produce serotonin and transform it into melatonin, and that many types of skin cells express receptors for both serotonin and melatonin.
In recent years, much attention has been devoted to the interaction between melatonin and the immune system.45, 46, 47 In numerous studies it has been found that melatonin supplementation increases cellular immunity. Specifically, supplemental melatonin increases T-lymphocyte proliferation48, 49; enhances antigen presentation by macrophages to T cells by increasing the expression of major histocompatibility complex class-II molecules50; activates splenic, lymph node and bone marrow cells51, 52; stimulates antibody-dependent cellular cytotoxicity53, and has been shown to augment both innate and adaptive immunity.54, 55, 56, 57 This research points to sunlight’s importance and beneficial effect on our immune system.
In conclusion, sunlight has a multitude of beneficial bioregulatory effects on our physiology. From the all-important production of vitamin D, to the release and control of nitric oxide, to the production of certain neuropeptides that heal tissues and modulate immunity, and the chronological regulation of melatonin with its effect on mental health and immunity, sunlight enlivens physiology. Of course, it is best to maintain caution with sunlight exposure to avoid the damaging effects of too much sun on the skin. However, research validates that some daily exposure to sunlight is essential for health and promotes longevity. Millions of people rarely seek sun exposure, and live in dwellings, offices and factories that have insufficient amounts of daytime light from windows. The implementation of restrictive sun exposure advice in countries with low solar intensity is now shown to be detrimental to longevity and cardiovascular health.
As the evidence grows that some sun exposure has benefits, many experts are rethinking their staunch sun-avoidance advice. A daily moderate dose of sunlight, without sunscreen, is as essential as food or water. As the 5th Dimension song goes, “Let the Sunshine, Let the Sunshine in.”
References
Wacker M and Holick MF: Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinol 5(1): 51-108, 2013.
Molozolowski W and Sniadecki J: (1786-1883) on the cure of rickets. Nature 143: 121, 1939.
Palm TA: The geographic distribution and etiology of rickets. Practitioner 45: 270-279, 321-342, 1890.
nach Finsen, Die Technik der Pockenbehandlung. Die Behandlung der Pocken durch Lichtentzug. Handbuch der Lichttherapie (1927): 430.
Rollier-Leysin, A. (1913). I. Die Heliotherapie der Tuberkulose. Ergebnisse der Chirurgie und Orthopädie, 7, 1.
Leuba, William. Die Heliotherapie der Fußtuberkulose. Deutsche Zeitschrift für Chirurgie 125, no. 5-6 (1913): 413-479.
Rollier, Auguste. Die Heliotherapie der Tuberkulose. Berlin: Springer, 1924.
Rollier, Auguste. Heliotherapy with Special Consideration of Surgical Tuberculosis. Heliotherapy with Special Consideration of Surgical Tuberculosis. (1927).
Rollier, Auguste. Heliotherapy: its therapeutic, prophylactic and social value. The American Journal of Nursing (1927): 815-823.
Roelandts, Rik. The history of phototherapy: something new under the sun? Journal of the American Academy of Dermatology 46, no. 6 (2002): 926-930.
Daniell, M. D., and J. S. Hill. A history of photodynamic therapy. Australian and New Zealand Journal of Surgery 61, no. 5 (1991): 340-348.
International Agency for Research on Cancer. IARC handbooks of cancer prevention, vol. 5: sunscreens. Lyon, France: International Agency for Research on Cancer, World Health Organization (2001).
American Cancer Society. Skin cancer facts. (2005).
US Department of Health and Human Services. The Surgeon General's call to action to prevent skin cancer. (2014).
Rigel, Darrell S. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. Journal of the American Academy of Dermatology 58, no. 5 (2008): S129-S132.
Apperly FL: The relation of solar radiation to cancer mortality in North America. Cancer Res 1:191–195, 1941.
Garland CF, Garland FC: Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 9:227–231, 1980.
Cuomo RE, Mohr SB, Gorham ED, Garland CF: What is the relationship between ultraviolet B and global incidence rates of colorectal cancer? Dermatoendocrinol 5:181–185, 2013.
Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC: Relationship between low ultraviolet B irradiance and higher breast cancer risk in 107 countries. Breast J 14:255–260, 2008.
Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC: Ultraviolet B irradiance and vitamin D status are inversely associated with incidence rates of pancreatic cancer worldwide. Pancreas 39:669–674, 2010.
Garland CF, Mohr SB, Gorham ED, Grant WB, Garland FC: Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer. Am J Prev Med 31:512–514, 2006.
Mohr SB, Gorham ED, Garland CF, Grant WB, Garland FC: Low ultraviolet B and increased risk of brain cancer: an ecological study of 175 countries. Neuroepidemiology 35:281–290, 2010.
Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC: Ultraviolet B irradiance and incidence rates of bladder cancer in 174 countries. Am J Prev Med 38:296–302, 2010.
Mohr SB, Gorham ED, Garland CF, Grant WB, Garland FC: Are low ultraviolet B and high animal protein intake associated with risk of renal cancer? Int J Cancer 119:2705–2709, 2006.
Moan, Johan, Alina Carmen Porojnicu, Arne Dahlback, and Richard B. Setlow. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proceedings of the National Academy of Sciences105, no. 2 (2008): 668-673.
Mohr SB, Garland CF, Gorham ED, Garland FC: Incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 51:1391–1398, 2008.
Simpson S Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B: Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry 82:1132–1141, 2011.
Rostand, Stephen G. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension 30, no. 2 (1997): 150-156.
Krause R, Bühring M, Hopfenmüller W, Holick MF, Sharma AM: Ultraviolet B and blood pressure. Lancet 352:709–710, 1998.
Kinney DK, Teixeira P, Hsu D, Napoleon SC, Crowley DJ, Miller A, Hyman W, Huang E: Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: a role for prenatal vitamin D deficiency and infections? Schizophr Bull 35:582–595, 2009.
Vieira VM, Hart JE, Webster TF, Weinberg J, Puett R, Laden F, Costenbader KH, Karlson EW: Association between residences in U.S. northern latitudes and rheumatoid arthritis: a spatial analysis of the Nurses' Health Study. Environ Health Perspect 118:957–961, 2010.
Geller, Alan C., Nina G. Jablonski, Sherry L. Pagoto, Jennifer L. Hay, Joel Hillhouse, David B. Buller, W. Larry Kenney et al. Interdisciplinary perspectives on sun safety. JAMA dermatology 154, no. 1 (2018): 88-92.
Holliman, Graham, Donna Lowe, Howard Cohen, Sarah Felton, and Ken Raj. Ultraviolet radiation-induced production of nitric oxide: a multi-cell and multi-donor analysis. Scientific reports 7, no. 1 (2017): 11105.
Liu, Donald, Bernadette O. Fernandez, Alistair Hamilton, Ninian N. Lang, Julie MC Gallagher, David E. Newby, Martin Feelisch, and Richard B. Weller. UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. Journal of Investigative Dermatology 134, no. 7 (2014): 1839-1846.
Feelisch, Martin. UVA Irradiation of Human Skin Vasodilates Arterial Vasculature and Lowers Blood Pressure Independently of Nitric Oxide Synthase. (2014).
Opländer, Christian, Christine M. Volkmar, Adnana Paunel-Görgülü, Ernst E. van Faassen, Christian Heiss, Malte Kelm, Daniel Halmer, Manfred Mürtz, Norbert Pallua, and Christoph V. Suschek. Whole body UVA irradiation lowers systemic blood pressure by release of nitric oxide from intracutaneous photolabile nitric oxide derivates. Circulation research 105, no. 10 (2009): 1031-1040.
Suschek, Christoph V., Christian Opländer, and Ernst E. van Faassen. Non-enzymatic NO production in human skin: effect of UVA on cutaneous NO stores. Nitric oxide 22, no. 2 (2010): 120-135.
Mourad, Jean-Jacques. The evolution of systolic blood pressure as a strong predictor of cardiovascular risk and the effectiveness of fixed-dose ARB/CCB combinations in lowering levels of this preferential target. Vascular health and risk management 4, no. 6 (2008): 1315.
MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J: Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 335:765–774, 1990.
Lindqvist, P. G., E. Epstein, Mona Landin‐Olsson, Christian Ingvar, Kari Nielsen, M. Stenbeck, and Håkan Olsson. Avoidance of sun exposure is a risk factor for all-cause mortality: results from the Melanoma in Southern Sweden cohort. Journal of internal medicine 276, no. 1 (2014): 77-86.
Taylor, A. W., D. G. Yee, T. Nishida, and K. Namba. Neuropeptide Regulation of Immunity: The Immunosuppressive Activity of Alpha‐Melanocyte‐Stimulating Hormone (α‐MSH). Annals of the New York Academy of Sciences 917, no. 1 (2000): 239-247.
Brenner, Michaela, and Vincent J. Hearing. The protective role of melanin against UV damage in human skin. Photochemistry and photobiology 84, no. 3 (2008): 539-549.
Scholzen, Thomas E., Thomas Brzoska, Dirk-Henner Kalden, Fiona O'Reilly, Cheryl A. Armstrong, Thomas A. Luger, and John C. Ansel. Effect of ultraviolet light on the release of neuropeptides and neuroendocrine hormones in the skin: mediators of photodermatitis and cutaneous inflammation. In Journal of Investigative Dermatology Symposium Proceedings, vol. 4, no. 1, pp. 55-60. Elsevier, 1999.
Klarsfeld, André, and Jean-Jacques d'Ortous. At the dawn of chronobiology. Technical report, ESPCI ParisTech, 2013.
Esquifino, Ana I., S. R. Pandi-Perumal, and Daniel P. Cardinali. Circadian organization of the immune response: a role for melatonin. Clinical and Applied Immunology Reviews 4, no. 6 (2004): 423-433.
Maestroni, Georges JM. The immunotherapeutic potential of melatonin. Expert opinion on investigational drugs 10, no. 3 (2001): 467-476.
Guerrero, Juan M., and Russel J. Reiter. Melatonin-immune system relationships. Current topics in medicinal chemistry 2, no. 2 (2002): 167-179.
Konakchieva, R., S. Kyurkchiev, Iv Kehayov, P. Taushanova, and L. Kanchev. Selective effect of methoxyindoles on the lymphocyte proliferation and melatonin binding to activated human lymphoid cells. Journal of neuroimmunology 63, no. 2 (1995): 125-132.
Raghavendra, V., V. Singh, A. V. Shaji, H. Vohra, S. K. Kulkarni, and J. N. Agrewala. Melatonin provides signal 3 to unprimed CD4+ T cells but failed to stimulate LPS primed B cells. Clinical & Experimental Immunology 124, no. 3 (2001): 414-422.
Pioli, Claudio, M. Cristina Caroleo, and Gino Doriac. Melatonin increases antigen presentation and amplifies specific and nonspecific signals for T-cell proliferation. International journal of immunopharmacology 15, no. 4 (1993): 463-468.
Wajs, Ewa, Eiji Kutoh, and Derek Gupta. Melatonin affects proopiomelanocortin gene expression in the immune organs of the rat. European journal of endocrinology 133, no. 6 (1995): 754-760.
Drazen, Deborah L., Donna Bilu, Staci D. Bilbo, and Randy J. Nelson. Melatonin enhancement of splenocyte proliferation is attenuated by luzindole, a melatonin receptor antagonist. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 280, no. 5 (2001): R1476-R1482.
Guerrero, Juan M., and Russel J. Reiter. Melatonin-immune system relationships. Current topics in medicinal chemistry 2, no. 2 (2002): 167-179.
Poon, Angela MS, Z. M. Liu, C. S. Pang, G. M. Brown, and S. F. Pang. Evidence for a direct action of melatonin on the immune system. Neurosignals 3, no. 2 (1994): 107-117.
Bonilla, Ernesto, Carolina Rodón, Nereida Valero, Héctor Pons, Leonor Chacín-Bonilla, Jorge García Tamayo, Zulay Rodríguez, Shirley Medina-Leendertz, and Florencio Añez. Melatonin prolongs survival of immunodepressed mice infected with the Venezuelan equine encephalomyelitis virus. Transactions of the Royal Society of Tropical Medicine and Hygiene 95, no. 2 (2001): 207-210.
Negrette, Beatriz, Ernesto Bonilla, Nereida Valero, Hector Pons, Jorge Garcia Tamayo, Leonor Chacín-Bonilla, Shirley Medina-Leendertz, and Florencio Añez. Melatonin treatment enhances the efficiency of mice immunization with Venezuelan equine encephalomyelitis virus TC-83. Neurochemical research 26, no. 7 (2001): 767-770.
Mead, M. Nathaniel. Benefits of sunlight: a bright spot for human health. (2008): A160-A167.
